INTRODUCTION
The outcomes of adolescent and young adult (AYA) patients (ages 15-39 years) with acute lymphoblastic leukemia/lymphoma (ALL) have improved in part with the adoption of pediatric ALL regimens in the adult treatment setting. The Cancer and Leukemia Group B (CALGB) 10403 trial (C10403) was the largest prospective trial conducted in the United States (U.S.), demonstrating the safety and efficacy of delivering an intensive pediatric regimen utilizing asparaginase in the adult oncology treatment setting to AYAs with ALL, with 3-year event-free survival (EFS) and overall survival (OS) of 59% (95% CI, 54%-65%) and 73% (95% CI, 68%-78%), respectively (Stock et al., Blood 2019). Although this regimen is widely used in the U.S., outcomes for patients treated with C10403 outside of a clinical trial setting remain unknown. We thus conducted a multi-center retrospective study to examine the real-world, off-trial efficacy of the C10403 regimen delivered across U.S. cancer centers.
METHODS
AYAs ages 17-40 years with newly diagnosed Ph-neg B-ALL or T-ALL between October 1, 2012 and July 1, 2020 who received initial treatment with the C10403 regimen (off trial) across 5 U.S. centers were included. Patients were excluded if they had Ph+ or Burkitt-type ALL. Previously treated patients, except with hydroxyurea, steroids, or one dose of single-agent therapy were excluded; patients given rituximab for CD20+ B-ALL were included. Primary endpoints were induction response rate (IRR), 3-year EFS, and 3-year OS. Induction response was defined as achieving <5% blasts on bone marrow by end of induction or extended induction. Patients were censored for EFS at the time of change in regimen, the addition of another agent (eg blinatumomab) or if they proceeded to hematopoietic cell transplant (HCT). Patients with deviation from the C10403 regimen were included in the 3-year OS analysis. Events were defined as: 1) failure to achieve bone marrow response by the end of induction or extended induction, 2) failure to achieve metabolic response of lymphoma on PET-CT as determined by the clinician 3) death, 4) relapse at any site, or 5) development of second malignancy.
RESULTS
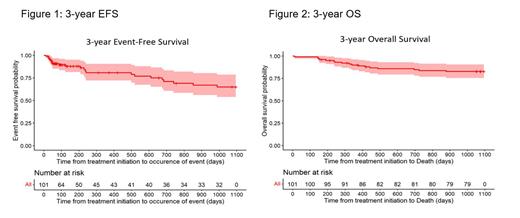
Among 101 included in the cohort, 54% were 20-29 years old, 69% were male, 55% were Caucasian and 31% identified as Hispanic. Of those with B-cell immunophenotype (70%), 49% had CD20 expression. The majority had normal karyotype (40%), while 3% were hypodiploid, 7% were KMT2a-rearranged, and 30% of the 27 patients assessed had Ph-like ALL. CNS involvement at diagnosis was documented in 20% (9% CNS2/11% CNS3) and 14% had a mediastinal mass. Of the B-ALL cohort (N=71), 16 (23%) received at least one dose of rituximab. Among the entire cohort, the IRR was 91% (54% MRD negative; threshold of at least 10 -4). Of the 101 patients who began induction with C10403, 72 (71%) completed induction and continued to consolidation; 51 (50%) continued beyond consolidation, and 31 (31%) completed the entire C10403 regimen through end of maintenance. Two patients (2%) died while in remission and still receiving treatment. Forty-four patients (44%) were taken off C10403 while in CR; 20 (20%) for allogeneic HCT, 23 (23%) for non-HCT alternative treatments including Hyper-CVAD or blinatumomab, and 1 (1%) for patient preference. The 3-year EFS and OS were 65% (95% CI, 53.8%-78.5%) and 82.7% (95% CI, 75.5%-90.5%), respectively (Figures 1 and 2).
Conclusion
In this multi-center, real world study of the C10403 regimen administered to newly diagnosed AYA ALL patients across adult U.S. centers, we demonstrate survival outcomes as favorable as the reported clinical trial results. While only 31% of patients completed the regimen through end of maintenance, a majority of patients who came off regimen either underwent consolidative HCT or alternative therapy while in CR. These data confirm the pediatric regimen as an excellent front-line option in AYA ALL.
Disclosures
Liedtke:Allogene: Other: Grants or contracts; BMS: Other: Grants or contracts; Participation on a Data Safety Monitoring Board or Advisory Board; Janssen: Other: Grants or contracts; Participation on a Data Safety Monitoring Board or Advisory Board; Abbvie: Other: Grants or contracts; Caelum: Other: Grants or contracts; Adaptive: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Seagen: Other: Grants or contracts; Kite: Other: Participation on a Data Safety Monitoring Board or Advisory Board. Stock:Kite: Consultancy; Kura: Research Funding; Servier: Other: Data Safety Monitoring Board/Advisory Board; Newave: Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; Glaxo Smith Kline: Consultancy; Amgen: Honoraria. Schwartz:Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy. Leonard:Kite/Gilead: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Takeda: Consultancy; Pfizer: Consultancy. Luskin:Novartis: Honoraria; Novartis: Research Funding; Pfizer: Honoraria; Jazz: Honoraria; AbbVie: Research Funding. Muffly:jasper: Research Funding; astellas: Consultancy, Research Funding; autolus: Consultancy; orca bio: Research Funding; pfizer: Consultancy; amgen: Consultancy; kite: Consultancy, Honoraria, Research Funding; bms: Research Funding; adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal